(12148 products available)


































































































































































































Oxidation sensors are devices used to measure the presence and concentration of oxidizing agents in various environments. These sensors are crucial in monitoring chemical processes, environmental conditions, and industrial operations where oxidation plays a significant role. There are several types of oxidation sensors, each designed to detect specific oxidizing agents or cater to particular applications. Here are some common types:
Chlorine sensors:
Chlorine sensors are used to monitor chlorine levels in water treatment processes, swimming pools, and industrial wastewater. These sensors ensure that chlorine levels remain within safe and effective ranges for disinfection and oxidation purposes.
Ozone sensors:
Ozone sensors measure ambient ozone levels, which is crucial for air quality monitoring and environmental research. These sensors help assess ozone's role as an oxidizing agent in the atmosphere and its impact on human health and the environment.
Peracetic acid sensors:
Peracetic acid sensors are used in the food and healthcare industries to monitor levels of this potent oxidizing disinfectant. These sensors ensure effective sanitation while preventing under- or overuse of peracetic acid in cleaning processes.
Hydrogen peroxide sensors:
Hydrogen peroxide sensors are employed in various applications, including food preservation, medical sterilization, and environmental monitoring. These sensors measure H2O2 levels to ensure that oxidation and disinfection processes are carried out effectively and safely.
Nitrate sensors:
Nitrogen compounds, such as ammonia and sulfide, can poison other types of sensors. Nonetheless, nitrate sensors are used in monitoring water quality and assessing the levels of nitrate ions, an essential nutrient for plant growth but can lead to eutrophication if present in excess. Oxidation sensors are used to convert ammonia to nitrate in biological processes.
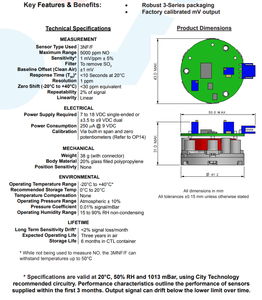
Here are some key specifications to help understand how oxidation sensors work:
Electrochemical Cell:
An oxidation sensor is an electrochemical cell that has 3 electrodes: a working electrode, a counter electrode, and a reference electrode. The electrodes are made from materials that facilitate oxidation-reduction reactions.
Operating Voltage:
Oxidation sensors operate at low voltages, typically between 0.5 to 1.0 volts. This voltage is used to monitor the reactions at the sensor's electrodes and not to initiate or drive the reactions.
Measured Species:
These sensors are designed to measure the concentration of oxidizing agents like ozone, chlorine, and hydrogen peroxide in various environments. Different sensors can be tailored to be selective toward specific oxidizing agents based on their electrochemical properties.
Response Time:
Oxidation sensors have a fast response time, typically ranging from seconds to minutes, depending on the design and the measured species. This allows for real-time monitoring of the concentration of the oxidizing agent in the environment.
Detection Limit:
These sensors have varying detection limits depending on the design and the measured species, typically ranging from nanomolar to micromolar concentrations. This enables sensitive detection and monitoring of oxidizing agents in various applications.
Temperature and Humidity Effects:
Oxidation sensors are designed to be affected by temperature and humidity. Some sensors have built-in compensation mechanisms to account for these environmental factors, ensuring accurate measurements in varying conditions.
Life Span:
These sensors have a limited lifespan, typically ranging from several months to a few years, depending on the design and the measured species. Environmental conditions, such as temperature and humidity, can also affect the sensor's lifespan.
Electrode Materials:
The materials used in the electrodes can significantly impact the sensor's performance and selectivity. Common materials include noble metals like platinum, gold, and silver, as well as transition metal oxides and conductive polymers.
The maintenance of oxidation sensors is important because it ensures optimal performance and longevity. Here are some maintenance requirements:
Regular inspection is important to ensure the sensor and associated equipment's physical and functional integrity. Check for signs of wear, damage, or corrosion and address any issues immediately.
Cleaning is important to remove dirt, dust, and other contaminants that could affect the sensor's performance. Use a soft brush or cloth and mild cleaning products to clean the sensor and its surrounding area.
Calibration ensures that oxidation sensors provide accurate and reliable readings. Follow the manufacturer's guidelines on the calibration frequency and procedure, and use certified reference materials for calibration.
Some parts of oxidation sensors, such as electrodes and membranes, may wear out over time and need to be replaced. Use original spare parts from the manufacturer to ensure compatibility and performance.
Environmental factors like extreme temperatures, humidity, and exposure to corrosive substances can affect oxidation sensors' performance and lifespan. Install the sensors in a suitable environment and take protective measures when necessary.
Follow the manufacturer's instructions and recommendations for maintaining oxidation sensors. This includes guidelines on installation, operation, and maintenance procedures, as well as safety precautions.
Choosing the right oxidation sensor for a specific application requires careful consideration of several factors to ensure optimal performance, reliability, and accuracy. Here are some key factors to keep in mind when selecting an oxidation sensor:
By considering these factors, selecting the right oxidation sensor for specific needs will be easier. Carefully evaluating the application requirements, reliability, durability, and performance of the oxidation sensor will help ensure optimal performance, reliability, and accuracy.
Here is a step-by-step guide on how to DIY and replace an oxidation sensor:
1. Preparation
Gather the necessary tools: users will need a wrench set, a ratchet and socket set, an oxygen sensor removal tool, and a torque wrench. Also, ensure the new sensor matches the vehicle's requirements. Ensure the vehicle is parked on a flat surface and the engine is cool before starting the replacement process.
2. Locate the sensor
Find the existing oxidation sensor in the exhaust system. Refer to the vehicle's manual for guidance. The guide will show the exact location of the sensor in the exhaust system and make the work easier.
3. Disconnect the electrical connector
Locate the electrical connector attached to the sensor and carefully disconnect it. This step is important to avoid any electrical short circuits while removing the sensor.
4. Remove the old sensor
Use the appropriate tool to loosen and remove the old oxidation sensor. In case it is stubborn, apply penetrating oil to help loosen it. Be careful not to damage the surrounding pipes or threads when removing the sensor.
5. Install the new sensor
Before installing a new sensor, clean the threads in the exhaust pipe where the sensor will be installed. Then, carefully install the new sensor into the exhaust pipe and tighten it to the recommended torque using a torque wrench. Be careful not to overtighten it, as this may damage the sensor or the exhaust pipe.
6. Reconnect the electrical connector
Connect the electrical connector to the newly installed sensor. Ensure it's securely attached to avoid any signal communication issues.
7. Final checks
Start the vehicle and let it run for a few minutes. Check for any exhaust leaks or unusual noises around the sensor area. Ensure the vehicle's monitoring system recognizes the new sensor and functions properly.
Q1: Where is the upstream oxygen sensor located?
A1: The upstream oxygen sensor, also known as the pre-catalytic converter O2 sensor, is situated before the catalytic converter, in the exhaust system. Its position allows it to detect the level of oxygen in the exhaust gases before they pass through the catalytic converter, helping the vehicle's computer regulate the air-fuel mixture for optimal emissions control and fuel efficiency.
Q2: What is the purpose of the 1st and 2nd oxygen sensors?
A2: The 1st oxygen sensor in a vehicle is responsible for monitoring the oxygen levels in the exhaust gases to help maintain an optimal air-fuel mixture, while the 2nd oxygen sensor, located after the catalytic converter, checks the efficiency of the catalytic converter by comparing its readings to those of the 1st sensor. This ensures proper emissions control and performance of the catalytic converter, contributing to overall vehicle efficiency and reduced environmental impact.
Q3: How many oxygen sensors does a car have?
A3: Most modern vehicles are equipped with two to four oxygen sensors. Typically, there are two sensors: one before and one after the catalytic converter, although some vehicles with larger engines may have additional sensors. These sensors play a crucial role in monitoring emissions and optimizing engine performance and fuel efficiency.
Q4: What is the difference between a lambda sensor and an oxygen sensor?
A4: While an oxygen sensor directly measures the levels of oxygen in the exhaust gases, a lambda sensor calculates the air-fuel ratio's closeness to being stoichiometric (ideal). In simpler terms, lambda sensors indicate whether the mixture is rich or lean, providing feedback to the engine control unit for adjustments. However, in many cases, the terms oxygen sensor and lambda sensor are used interchangeably, as both serve similar functions in emissions control and engine performance.